LIBS Technology, the identity of Ablatom:
The analytical solutions developed and distributed by Ablatom are based exclusively on LIBS technique (Laser-Induced Breakdown Spectroscopy) and allow to detect, identify, localize and/or even quantify the chemical elements of a sample.
The science behind LIBS:
LIBS is an elemental analysis technique whose principle combines laser ablation and atomic emission spectroscopy to characterize the chemical composition of any sample. A focused laser pulse ablates a small fraction (few nanogram) of the material and excites the chemical elements on the surface of the sample producing a plasma.
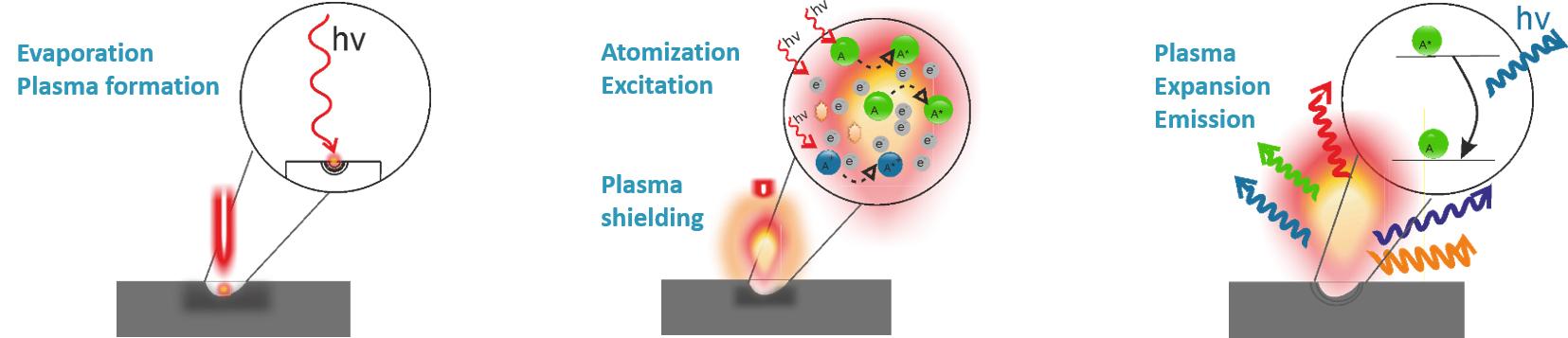
The optical emission of such plasma is collected as a spectrum. This LIBS spectrum contains information about what elements and in which amount are present in the sampling point. Spectroscopically, one can study the sample composition both in a qualitative and/or quantitative way by the use of standards or calibration methods.
Among all the different types of atomic emission spectroscopy techniques, LIBS is the unique technique able to vaporize, excite and atomize in a single process (i.e., a laser pulse). When the excited electrons return to the ground state in a process called electronic relaxation, all that extra energy is released as photons. This emission is then collected and analyzed with an optical spectrometer producing a unique pattern of lines separated into different wavelengths (spectrum) characteristics of the sample composition.
Why you should use LIBS? Featured advantages:
In few milliseconds (laser pulse, plasma formation and light collection) a LIBS spectrum produces enough information to characterize the elemental composition of a sample. But there is more, LIBS can analyse all type of samples no matter what elements are made up (even light elements) with an all-optic instrumentation, without contact and just using few nanogram of material per pulse. Not many analytical techniques could say the same, so if you look for a fast, reliable and simple technology, LIBS becomes a serious candidate.
Any element is detectable by LIBS. There are no limitations in regard to the atomic number, H, Li, Be, B, C are easily detectable by LIBS. Luckily most of them emit strong and isolated lines detectable.
These are unique characteristics that enable the development of novel applications from laboratory to industry in a range of fields from geology to material science as well as quality control industry, aerospace or energy science.

Solutions to Industry:
Inherently, LIBS technique does not require direct contact with the sample nor previous preparation processes to make elements accessible. For industrial processes this is a dream that comes true. LIBS allows literally bringing the laboratory to the sample and not the other way round enabling real-time monitoring of chemicals in continuous processes. Combining LIBS with either multivariate analysis (i.e., chemometrics), or univariate analysis (i.e. spectral feature tracking) would help you to increase the knowledge of your process and will lead to an increase in the production yield and profit.
Ablatom provides years of expertise on the development to tailor-made solution to transform LIBS technology into an industrial analyser for metallurgy, mining and a range of industries.
MicroLIBS Imaging at the state of art:
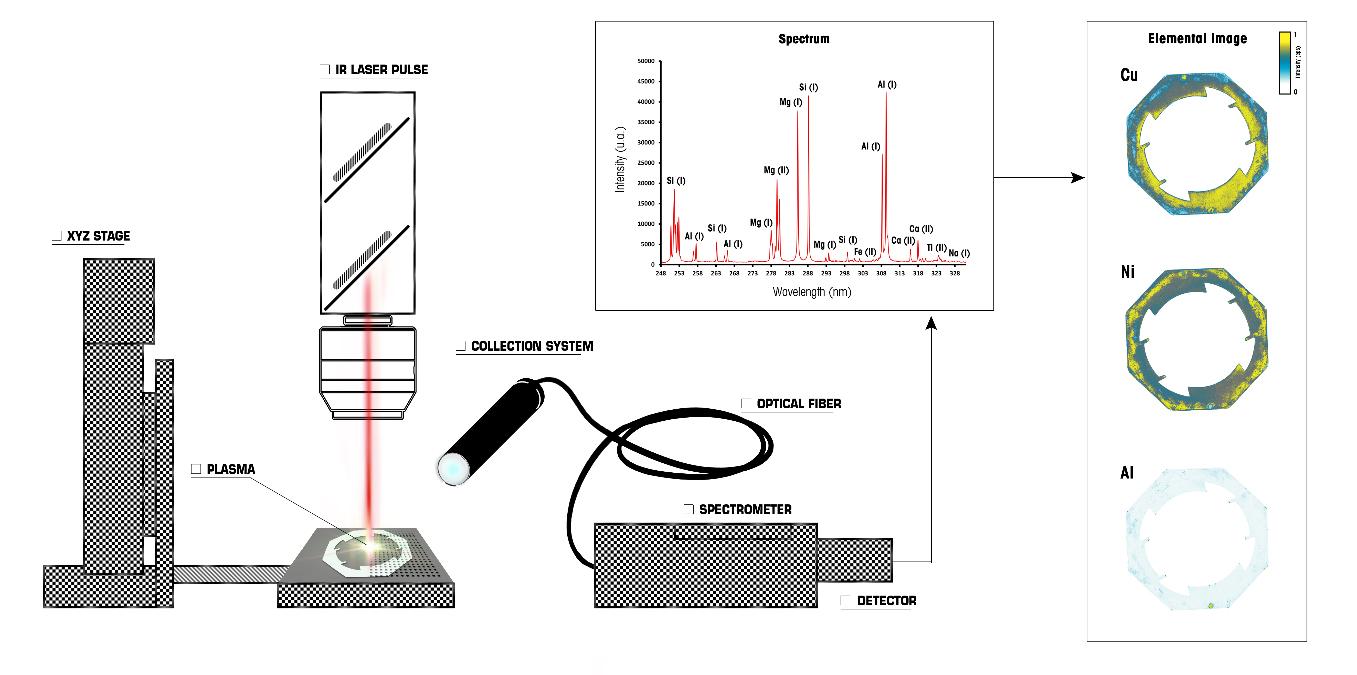
LIBS microscopy allows to generate chemical images giving the spatial distribution of different species on a given surface with a microscale spatial resolution. The principle of LIBS imaging, a term introduced by our historical research partner (ILM), is based on the generation of a series of plasma at different successive positions on the surface being analyzed. In the most common configuration, the surface is moved under the beam at a speed synchronized with the rate of the laser pulses, which can reach up to 1000 measurement points per second. It is thus possible to reconstruct a set of hyperspectral data in two or three dimensions. Each laser shot corresponds to a pixel of the final image and gives an emission spectrum representative of the composition of the material at that point. A single LIBS image therefore contains chemical information from several hundred thousand to several million spectra. The result of a single LIBS analysis can be composed of as many chemical images of the analyzed surface as there are emission lines detected.
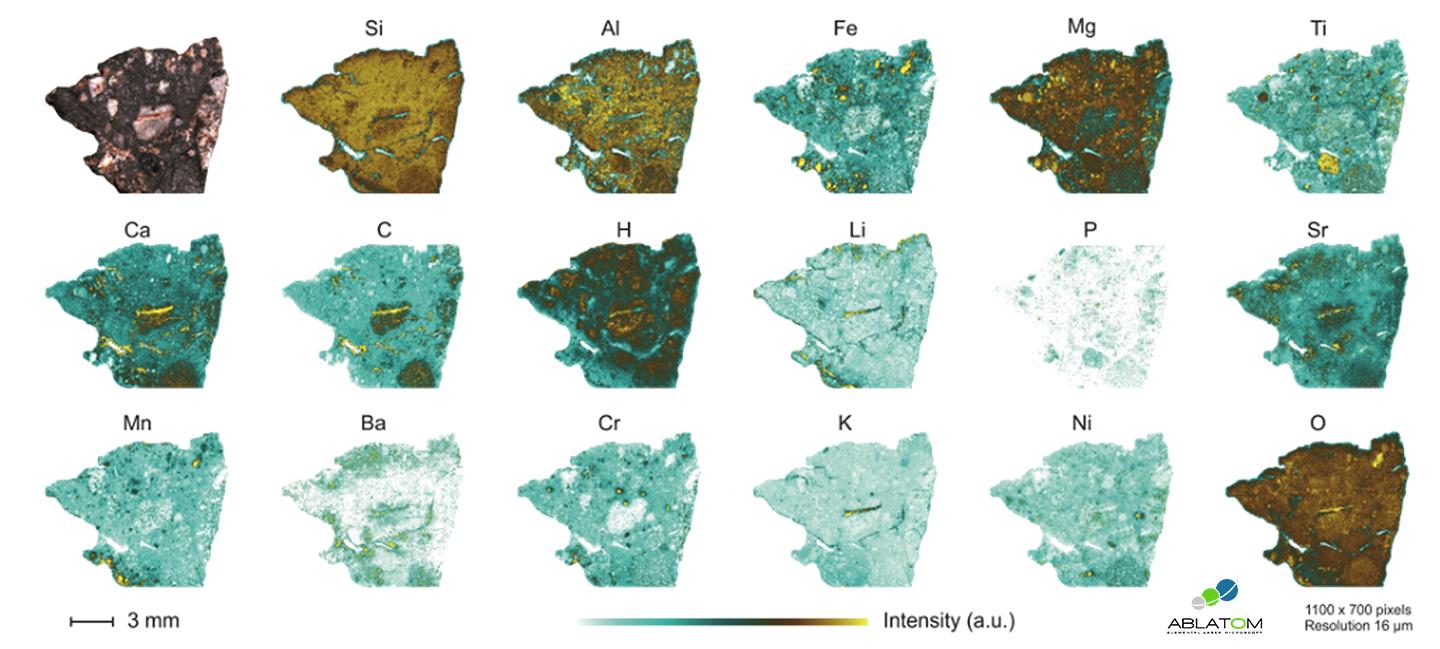
Its high speed of analysis, combined with a sensitivity in the ppm range and a spatial resolution at the micrometer scale, make LIBS imaging revolutionary and already represents an powerful elemental imaging tool in geosciences, biology or even materials science.
